Research
|
Organisms must respond to their environment
The aim of the research in my lab is to elucidate mechanisms that underlie the ability of organisms to successfully develop and function in the face of environmental challenges. Years of research have given us insight into how organisms develop and function under controlled laboratory settings; however, outside the lab organisms experience a range of environmental challenges, many of which may lead to organismal dysfunction and disease. Very little research has been done on how cellular processes responsible for proper development and function respond to environmental change. Therefore, more exploration is needed to broaden our understanding of what organisms can do in the face of environmental challenges to maintain proper function and also what can go wrong when this does not happen. My research uses C. elegans to investigate the mechanisms that promote successful development and organismal function in the face of changing environmental conditions, specifically changing temperature. |
|
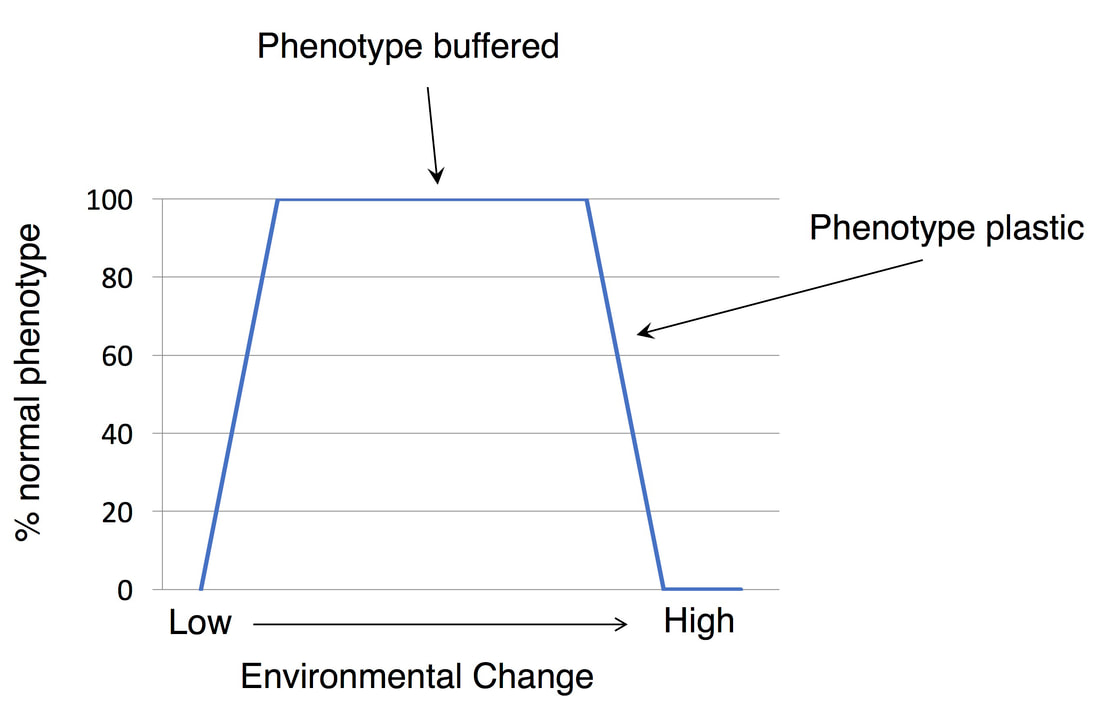
Buffered or Plastic: Organisms can respond to environmental change in two ways
How an organism responds to changes in the environment is described in two ways. When function or development is not affected by changes in the environment then that organism is buffered to that environmental factor. During buffering the organism employs mechanisms that allow it to respond so that there is no detrimental outcome. However, there is generally a point at which the environmental challenge is so great that function or development begins to be compromised; at this point the organism’s response to the environment is called plastic. The same organisms can have both buffered and plastic outcomes depending on the environmental range. For example, C. elegans maintains fertility between 12°C and 26°C, demonstrating a buffered system, but becomes sterile above 26°C, thus demonstrating plasticity. My lab has two research focuses where we study both of these types of responses. |
Buffering of cell fate by the DREAM complex and LIN-15B
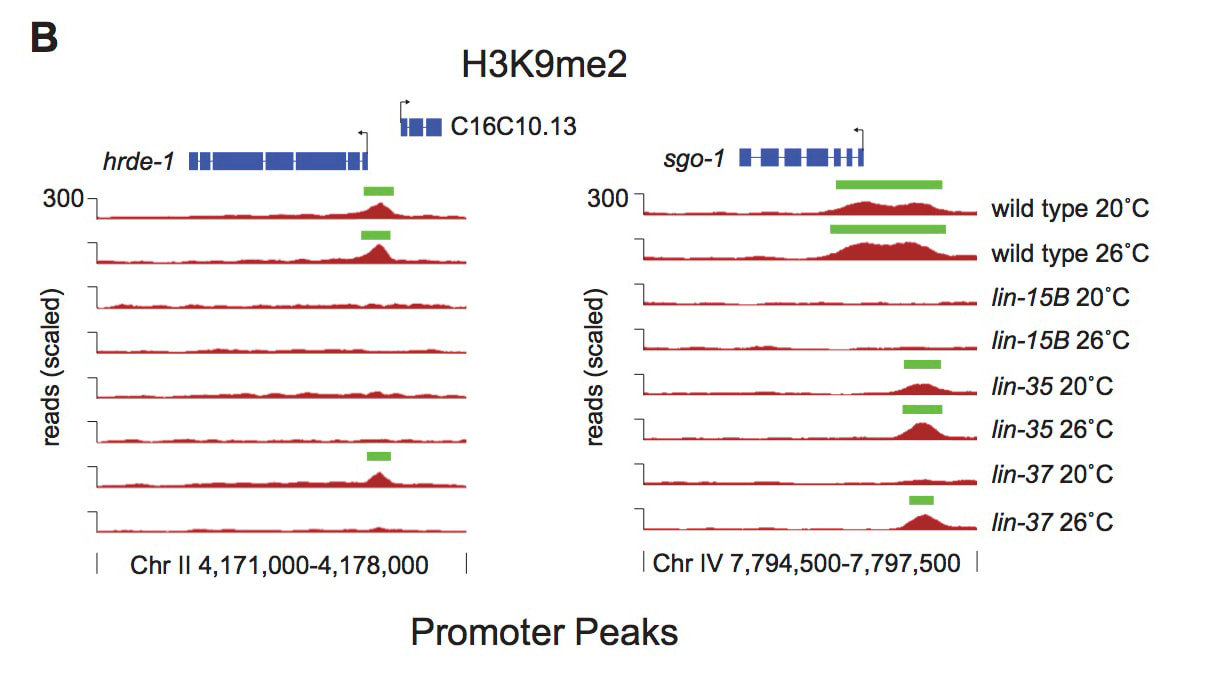
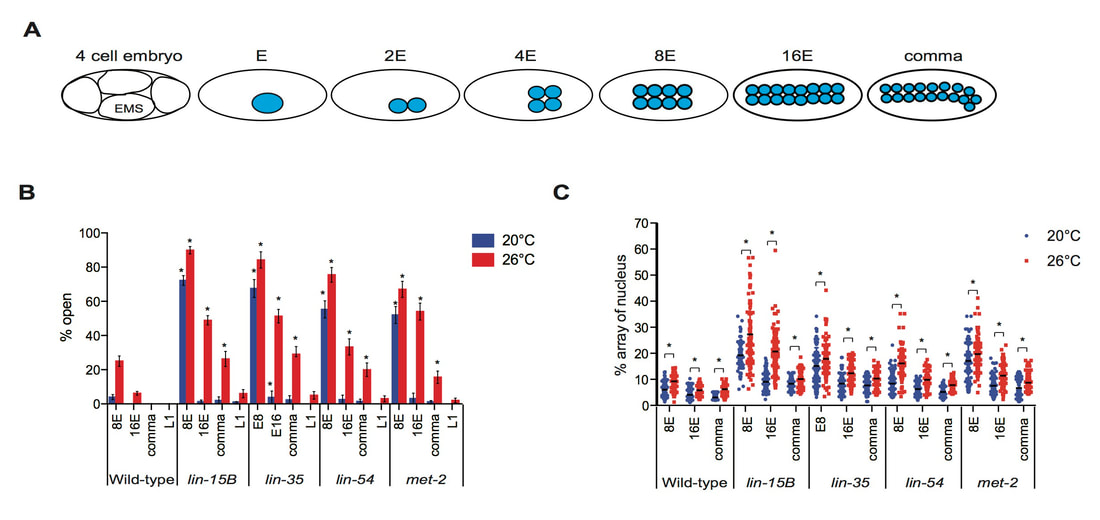
The DREAM complex is a conserved transcriptional repressor that we have shown works with LIN-15B to repress expression of genes in the soma that are normally only expressed in the germline. DREAM complex or lin-15B mutants show expression of these germline genes in somatic tissues. Interestingly, ectopic germline gene expression is enhanced by growing worms at 26°C, which results in a high temperature larval arrest phenotype. Our recent work has shown that LIN-15B is necessary for the localization of a repressive histone modification, trimethylation of histone H3 lysine 9, on the promoters of germline genes (Rechtsteiner et al, 2019). However, this modification is lost in lin-15B mutants at both 20°C and 26°C and thus cannot underlie the temperature sensitivity of mutants. Instead, our work has shown that the temperature sensitivity of mutants is due to changes in the timing of chromatin compaction during embryonic development (Costello and Petrella, 2019). We have shown that in DREAM complex or lin-15B mutants normal compaction of chromatin surrounding germline gene loci is delayed, and then open chromatin is maintained in mutants, but only at 26°C. Our work suggests a new model of tissue specific gene repression during development, where repressive factors are necessary to create compact chromatin environments around genes that are not destined to be expressed in a lineage. If this compaction is delayed due to the combined loss of repressive factors and elevated temperature, then these genes are vulnerable to expression when the maternal to zygotic switch occurs. We are currently investigating what pathways are necessary to drive the expression of ectopic germline gene expression in the absence of these repressive factors. We have found that the planar cell polarity WNT signaling pathway is one of the strongest drivers of ectopic germline gene expression, and are currently determining how and when this signaling plays a role in these phenotypes during development.
The DREAM complex is a conserved transcriptional repressor that we have shown works with LIN-15B to repress expression of genes in the soma that are normally only expressed in the germline. DREAM complex or lin-15B mutants show expression of these germline genes in somatic tissues. Interestingly, ectopic germline gene expression is enhanced by growing worms at 26°C, which results in a high temperature larval arrest phenotype. Our recent work has shown that LIN-15B is necessary for the localization of a repressive histone modification, trimethylation of histone H3 lysine 9, on the promoters of germline genes (Rechtsteiner et al, 2019). However, this modification is lost in lin-15B mutants at both 20°C and 26°C and thus cannot underlie the temperature sensitivity of mutants. Instead, our work has shown that the temperature sensitivity of mutants is due to changes in the timing of chromatin compaction during embryonic development (Costello and Petrella, 2019). We have shown that in DREAM complex or lin-15B mutants normal compaction of chromatin surrounding germline gene loci is delayed, and then open chromatin is maintained in mutants, but only at 26°C. Our work suggests a new model of tissue specific gene repression during development, where repressive factors are necessary to create compact chromatin environments around genes that are not destined to be expressed in a lineage. If this compaction is delayed due to the combined loss of repressive factors and elevated temperature, then these genes are vulnerable to expression when the maternal to zygotic switch occurs. We are currently investigating what pathways are necessary to drive the expression of ectopic germline gene expression in the absence of these repressive factors. We have found that the planar cell polarity WNT signaling pathway is one of the strongest drivers of ectopic germline gene expression, and are currently determining how and when this signaling plays a role in these phenotypes during development.
|
Loosing fertility at high temperature
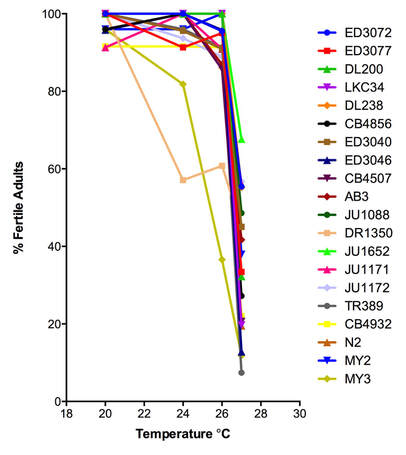
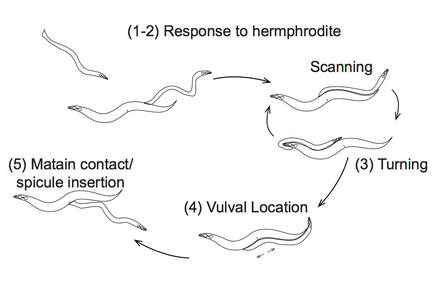
Sensitivity of the germline to elevated temperature is conserved from worms to humans. As a way of investigating the molecular mechanisms that underlie high-temperature sterility and to determine pathways that could be utilized to buffer fertility at high temperature, we are studying the natural variation in germline temperature sensitivity in wild-type isolates of C. elegans. Our study of 20 wild-type isolates from around the world demonstrated a range of population fertility (from 7%-67%) at 27°C (Petrella, 2014). All strains showed some loss of sperm function. However, there were two novel discoveries in our data. 1) There is great variability in the level of loss of oogenic germline function at high temperatures. 2) There is great variability in the ability of male worms to recover fertility when moved from high temperatures to lower temperatures. Follow-up research using male wild type strains has demonstrated that the primary defect leading to loss of fertility in males at 27°C is behavioral: males are no longer attracted to hermaphrodites even when exposed to 27°C for only 24 hours (Nett et al., 2019). Our ongoing studies are using a larger number of wild type C. elegans strains to determine which aspects of germ cell function are sensitive to temperature. We have completed analysis of fertility at 27°C in 92 wild type strains, and have found significantly associated loci using a GWAS. Additionally, we are investigating if there are differences in gene expression in these strains, which may underlie their different high-temperature phenotypes. Finally, using a number of microscopy and molecular techniques, we are investigating the changes to oocytes and sperm that occur at high temperature that may lead to loss of fertility at elevated temperatures. A new research project in the lab merges our two research interests in investigating both DREAM complex function and fertility. We have demonstrated that the DREAM complex is necessary for the increased level of germline apoptosis seen in wild type hermaphrodites exposed to 26°C. We are currently investigating how the DREAM complex plays a role in buffering oocyte quality at 26°C. |
Proudly powered by Weebly